In November 2020, the Center for Genetic Engineering and Biotechnology of Cuba launched a trial on a coronavirus vaccine called Abdala. The Abdala vaccine consists of a piece of the coronavirus spike protein called the receptor binding domain, and is delivered in three doses. On Feb. 1, the center held a press conference to announce the start of a Phase 2 trial. A Phase 3 trial involving up to 48,000 participants was launched on March 18. On May 12, while the Phase 3 trial was still underway, the Cuban government began rolling out Abdala in a mass vaccination campaign, in the hopes of reining in a surge of cases. On June 21, Cuban officials reported that Abdala had an efficacy of 92.28 percent.
The Cuban government granted emergency use authorization for the vaccine on July 9. Abdala is one of two Cuban vaccines tested in a Phase 1/2 clinical trial to assess their ability to increase immunity in those who have already had Covid-19. Starting in August 2020, CanSino began running Phase 3 trials in a number of countries, including Pakistan, Russia, Mexico and Chile. On Feb. 25, China announced the approval of the CanSino vaccine for general use. The company announced that its one-shot vaccine had an efficacy rate of 65.28 percent at preventing all symptomatic Covid-19 cases.
But on April 1, CanSino's chief scientific officer said that the efficacy of its vaccine could drop over time. He also floated the idea of using a booster shot six months after the first dose, though more clinical trial data is needed. Reuters reported on Aug. 5 that this drop could be as much as around 30 percent after six months.
Preliminary findings from its phase one trials showed that healthy subjects—including elderly patients—produced coronavirus antibodies and a reaction from T cells, another part of the human immune response. The company also announced plans to test the safety and efficacy of a booster shot that would be delivered a year after the first pair of vaccine doses, according to CNBC. On March 23, CanSino announced that it had won approval for a clinical trial of an inhaled version of the vaccine.
Chief Executive Yu Xuefeng said on June 2 that this version is now in Phase 2 trials. Preliminary findings from the Phase 1 trial suggest that the inhaled vaccine was effective at stimulating an immune response, and that an injected dose followed by an inhaled dose may yield the best results. Researchers have also begun to test whether giving alternating doses of vaccines from CanSino and Anhui Zhifei Longcom can boost their effectiveness. On June 9, Reuters reported that the CanSino vaccine is being tested as a booster shot.
Results from a Chinese study, released on Sept. 7, suggested that getting the CanSino booster shot after the Sinovac vaccine produced a stronger antibody response compared to a third shot of the Sinovac vaccine. CanSino announced on Sept. 26 that a trial in children age 6 to 17 yielded positive results. On June 1, Moderna announced that it has applied for full FDA approval of its COVID-19 vaccine for use in people age 18 and older. The company also plans to file for emergency use authorization for teens ages 12 to 17. A study of clinical trials among adolescents in this age group shows that its vaccine is safe and 100 percent effective. The study also showed the vaccine is 93 percent effective among participants in this age group two weeks after the first dose.
Researchers at City of Hope, a California biomedical research institute, created a vaccine based on a weakened form of a virus called Modified Vaccinia Ankara, or MVA for short. They added two coronavirus genes to the MVA virus — one for the spike protein, and one for another protein called nucleocapsid. They are testing the vaccine specifically for people with immune systems impaired by cancer and other disorders. Many of them do not produce a strong immune response to authorized vaccines based on mRNA. In September 2021, researchers launched a Phase 2 trial, giving the vaccine to volunteers with blood cancer who have received a bone marrow transplant or a form of immunotherapy called CAR-T.
Before the pandemic, the Pennsylvania-based company Inovio developed DNA-based vaccines that are delivered into the skin with electric pulses from a hand-held device. They are running clinical trials for vaccines against a number of diseases, including HIV, Zika, and several forms of cancer. At the start of the pandemic, Inovio developed a DNA vaccine against the spike protein on the coronavirus. A Phase 1 trial, published in December, did not uncover any serious adverse effects, and measured an immune response in all 38 volunteers.
Although progress has been made over the past decades in clinical trial transparency, and there are some successes for COVID-19 vaccines, there is still much room for improvement. The lack of adequate transparency about COVID-19 vaccine trials and their regulation cannot be dismissed as unfortunate, stubborn problems emblematic of the present culture in biomedicine. In a time of increasing public scrutiny, transparency of regulatory decision making leading to the approval of drug treatments and vaccines for COVID-19 is important to ensure patient and stakeholder trust. It is a scientific, moral and ethical imperative that access to complete trial data of these global public health interventions is urgently granted to patients, researchers and other key stakeholders. Finally, clinical trials occurred at different times and in different countries — which means that the prevalence of viral variants also differed. Pfizer-BioNTech's and Moderna's vaccines were tested before variants of concern were widely circulating, making it harder to know how effective they are against variants.
Three months agowe described the existing data on vaccines and variants, including the lack of clinical trial evidence for most vaccine/variant combinations. Studies fromEnglandandScotlandsuggest Pfizer and AstraZeneca vaccines offer somewhat reduced protection against infection by the highly transmissible Delta variant, but similar protection against severe illness. On Nov. 5, Turkey's Erciyes University announced they had begun injecting volunteers with an inactivated coronavirus vaccine called ERUCOV-VAC. It was the first clinical trial of a coronavirus vaccine developed in Turkey.
On Dec. 14, 2020, the president of the university said that the Phase 1 trial was complete. On June 23, 2021, Turkish president Recep Tayyip Erdoğan announced that the vaccine, renamed Turkovac, has entered a Phase 3 trial. In July, the researchers registered a Phase 2 trial to evaluate the vaccine as a booster shot. The Chinese company CanSino Biologics developed Convidecia in partnership with the Institute of Biology at the country's Academy of Military Medical Sciences. Last May, researchers published promising results from a Phase 1 safety trial on Convidecia, and in July they reported that their Phase 2 trials demonstrated the vaccine produced a strong immune response.
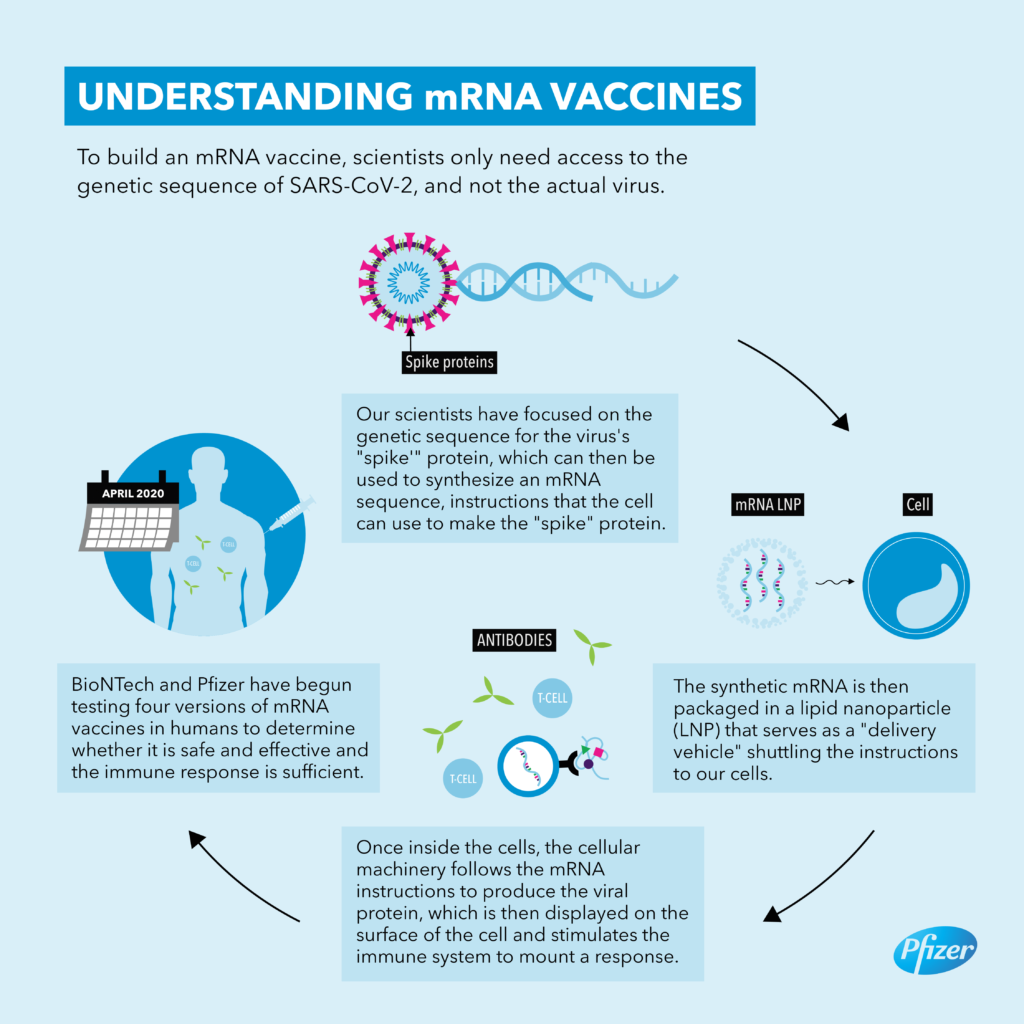
On Nov. 9, 2020, New York-based Pfizer and the German company BioNTech made history by announcing that their coronavirus vaccine had an efficacy rate of over 90 percent, far surpassing expectations. Just over a month later, on Dec. 11, the Food and Drug Administration granted the vaccine, known as Comirnaty, the first emergency use authorization ever given by the United States to a coronavirus vaccine. On Aug. 23, 2021, the F.D.A. granted full approval to the Pfizer-BioNTech vaccine, for people 16 and older, and on Sep. 22, the agency authorized a third dose as a booster for people age 65 and older, and other populations at high risk.
It is also 85 percent effective in people 65 and older and 100 percent effective at preventing severe cases of the disease. The company also said that real-world evidence and data from its clinical trials show that its single-dose vaccine remains effective against COVID-19. The press release cited a study published to a preprint server, which shows that the vaccine remains 79 percent effective in preventing COVID-19 and 81 percent effective in preventing hospitalizations. Johnson & Johnson said these data are consistent with findings from its clinical trials, which showed 75 percent efficacy against severe disease and 89 percent efficacy against hospitalization. Researchers at the Institute of Medical Biology at the Chinese Academy of Medical Sciences, which has invented vaccines for polio and hepatitis A, created an inactivated coronavirus vaccine. In May 2020, they launched a Phase 1 trial on 192 volunteers which indicated the vaccine was safe and produced an immune response.
A Phase 2 trial followed on 750 volunteers, which led the researchers to select a two-week spacing between the two doses of the vaccine. In December the researchers launched a Phase 3 trial on up to 34,020 volunteers in Brazil and Malaysia. On June 9, Chinese government newspaper Science and Technology Daily reported that the vaccine received emergency use authorization.
On Dec. 30, Sinopharm announced that the vaccine had an efficacy of 79.34 percent, leading the Chinese government to give it approval. On May 7, 2021, the World Health Organization put forward a similar efficacy estimate of 78.1 percent and gave the vaccine emergency use authorization. On Aug. 3, Bloomberg reported that results from a Hungarian study showed that the vaccine failed to produce enough antibodies in more than one quarter of elderly people.
Results from a Peru trial suggest that the vaccine was 50.4 percent effective in preventing infections among healthcare workers, Reuters reported on Aug. 13. On Sept. 11, 2021, COVAXX registered a Phase 1 trial in Taiwan which led to 100 percent of volunteers producing antibodies without any serious side effects. A Phase 2/3 trial is planned to launch in Brazil, India and other countries. On Nov. 25, Covaxx announced agreements with countries including Brazil, Ecuador, and Peru to deliver more than 140 million doses for $2.8 billion. In January, the company announced they were also starting preclinical research on a vaccine tailored specifically to newly emerged coronavirus variants that could potentially evade conventional vaccines.
In a June 21 press release, Vaxxinity said it expected to deliver the vaccine by the end of the summer, which did not come to pass. The researchers registered a Phase 1 trial on July 20 to assess the effectiveness of a third dose of its vaccine in those who have already received two. But in August, Taiwan regulators rejected an application for emergency approval of the vaccine after it failed to meet their standards on neutralizing antibodies. Last spring, researchers at the University of Washington developed a nanoparticle studded with pieces of the coronavirus spike protein.
The South Korean vaccine company SK Bioscience licensed the vaccine, called GBP510. After partnering with GSK, they launched a Phase 1/2 trial of the vaccine in February 2021. It is the second vaccine SK Bioscience has put into trials, after launching a study on another protein-based vaccine called NBP2001. SK Bioscience has received $210.1 million from the Coalition for Epidemic Preparedness Innovations for the development of GBP510.
In August, the company launched a Phase 3 trial, comparing GBP510 to AstraZeneca's Vaxzevria vaccine. Phase III clinical trials are critical to understanding whether vaccines are safe and effective. In Phase III, participants and most of the study investigators do not know who has received the vaccine and who received the placebo.
Participants are then followed to see how many in each group get the disease. Assessing short- and long-term safety is also a major goal of phase 3 trials. Phase II clinical trials continue to assess safety and immune responses but in a larger number and more diverse group of volunteers, typically one to several hundred people. Phase II trials may include target populations of a specific age or sex, or those with underlying medical conditions.
Vaccines for children start with adult volunteers and move to progressively younger groups of children. Different types of immune responses are often measured, including antibodies and cell-mediated immunity, but phase II trials do not assess how well a vaccine actually works. Additionally, there is a need for transparency around any deliberations regarding the length of follow-up necessary to adequately assess efficacy and safety prior to licensure.
Pfizer has already submitted its initial data on the safety and efficacy of its vaccine for children in that age group. The company said at the time that an analysis of its clinical trials of 2,268 participants shows the vaccine is safe and elicits "robust" neutralizing antibodies. The vaccine follows a two-dose regimen of 10 milligram doses—a third of the dosage that is given to adults. The company said at the time that an analysis of its clinical trial of 2,268 participants shows the vaccine is safe and elicits "robust" neutralizing antibodies. The Chumakov Center at the Russian Academy of Sciences developed an inactivated coronavirus vaccine called CoviVac.
On Oct. 14, 2020, Tass reported that clinical trials of the vaccine would begin in Kirov and St. Petersburg on Oct. 19. On Feb. 20, 2021, Russia approved the vaccine for domestic use, despite the fact that the Chumakov Center only later began a Phase 3 trial. On June 3, the director of the Chumakov Center said the trial was still underway and it was not yet possible to speak of the vaccine's efficacy.
The director said on Aug. 24 that the Chumakov Center is working on a modified version of the vaccine tailored for new variants, and that they plan to double the production capacity for CoviVac to 2.5 million doses per month. The director told Tass in October that a trial for adults aged 60 and older could begin soon. Shafa Pharmed Pars, an Iranian pharmaceutical company, developed a vaccine made of inactivated coronaviruses. Results from preclinical trials showed that the vaccine was safe and effective in animals. Known as COVIran Barekat, it entered a Phase 1 trial at the end of December, becoming the first vaccine developed in Iran to go into clinical testing.
COVIran Barekat began a Phase 3 trial on April 25, 2021, and on June 14, the Iranian government announced it had authorized the vaccine, despite the fact that many volunteers in the Phase 3 trial had not yet received their second dose. On June 25, Ayatollah Khamenei received the COVIran Barekat vaccine on television. As part of the European Union-funded PREVENT-nCoV consortium, a team of biotechnology companies and research laboratories developed a vaccine against Covid-19. The vaccine, called ABNCoV2, uses technology from consortium members AdaptVac and ExpreS2ion, among others. After promising preclinical results in primates, Bavarian Nordic announced that it would proceed with a Phase 1/2 trial of the vaccine in the Netherlands. On Aug. 9, the researchers said that the trial showed ABNCoV2 produced high levels of antibodies without dangerous side effects.
Later that month, Bavarian Nordic launched Phase 2 trials of the vaccine, both as an initial protection against Covid-19 and as a booster for other vaccines. Maryland-based Altimmune is a biopharmaceutical company that focuses on developing vaccines delivered by nasal spray. They developed a nasal spray vaccine for Covid-19, delivering the Ad5 adenovirus to the airway. Studies on the immune system suggests that a nasal spray could be more effective for blocking the transmission of the virus than vaccines given by injection.
In a study on mice, Altimmune researchers found that a single dose of the vaccine gave complete protection from a lethal infection of coronaviruses. On Dec. 22, 2020, the company registered a Phase 1 clinical trial of a single dose of the vaccine. DISTRIBUTIONWhile Moderna's clinical trials were still underway in the summer of 2020, the company entered deals with several countries to supply the vaccine pending its approval. On Aug. 11, the United States government awarded the company $1.5 billion in exchange for 100 million doses if the vaccine proves safe and effective. Additional negotiations have increased the agreement to 500 million doses.
Moderna has made similar deals with other countries including Canada, Japan, Qatar and South Korea. The company has also pledged 500 million doses to COVAX, a global vaccine initiative, to supply vaccines to low-income countries. On April 29, Moderna announced they would produce 800 million to 1 billion doses in 2021, and planned to manufacture 3 billion doses in 2022. Phase 2 clinical trials are conducted with larger and more targeted populations — for instance, people who may be at risk for a specific infection. If the study vaccine is considered safe and the immune system responds as expected, then the study vaccine moves into phase 3.
Florida's coronavirus death toll hit 10,000 on Wednesday as the state continues to struggle to get the ongoing pandemic under control. Almost six months since Florida's first case was identified, the state reported 174 deaths on Wednesday, bringing the total death toll to 10,067. The state reported 584,047 Covid-19 cases on Wednesday, up 0.7% from a day earlier, compared with an average increase of 1% in the previous seven days.
The new daily rate of people testing positive for the first time fell to 7.1% for Tuesday, the lowest since June 14. The state has reported 33,146 new cases in the past seven days, the fewest in a comparable period since late June. Vaccines differed substantially in the amount of vaccine per dose in clinical trials — and consequently in the amount of vaccine given to patients. For instance, Moderna vaccines were tested with 100 micrograms of mRNA per dose, while Pfizer-BioNTech was tested with 30 micrograms per dose.






















No comments:
Post a Comment
Note: Only a member of this blog may post a comment.